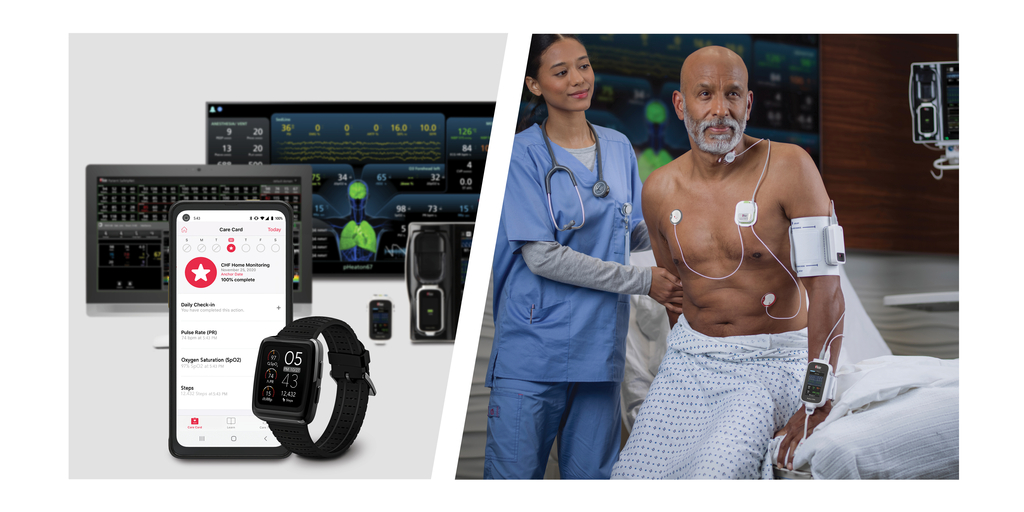
Scope of the SESHAT Project at Pioneering Hospital, Part of the Catalan Health Institute, Includes Deployment of up to 1,000 Masimo W1 Watches, 100 Radius VSMs, and Numerous Additional Masimo Hospital Automation™ Solutions
BARCELONA, Spain–(BUSINESS WIRE)–Masimo (NASDAQ: MASI) today announced that Germans Trias i Pujol, a pioneering hospital serving more than 800,000 people in north Barcelona, Spain, is launching a breakthrough telehealth and remote patient management initiative using Masimo technology. The SESHAT project is focused on using advanced wearable technologies and wireless connectivity to allow remote clinicians to keep track of patients’ physiological data in near real time wherever they are, whether the patient is in another part of the hospital or at home. The project, which began in the fourth quarter of 2023 and is slated to last at least three years, includes the implementation of up to 1,000 Masimo W1® medical watches, 100 Radius VSM™ Wearable Continuous Vital Signs Monitors, 10 Patient SafetyNet™ Systems, and a host of additional Masimo Hospital Automation™ products.
Joe Kiani, Founder and CEO of Masimo, said, “We are honored to partner with Germans Trias i Pujol to support the SESHAT project. When I founded Masimo 35 years ago, our mission was to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. Now, with decades of innovation and refinement, we are bringing our pioneering accurate and continuous monitoring technologies to even more new sites and applications, including the home – with powerful wearable, tetherless, and remote modalities like Masimo SET®-based Masimo W1 and Radius VSM. Our breakthrough predictive algorithm Halo™, AI-powered tools, and connectivity and automation solutions in turn make that accurate, precise, rich stream of actionable patient data available to clinicians when and where they need it, helping patients feel connected and cared for, and informing evermore insightful decisions about care. Ultimately, we believe this will improve outcomes while reducing the cost of care, in keeping with our founding mission. We look forward to gleaning innumerable insights and improving 800,000 lives with the forward-thinking clinicians of Germans Trias i Pujol.”
Dr. Oriol Estrada, Director of Healthcare Strategy and Innovation at Germans Trias, commented, “Project SESHAT, named for the Egyptian goddess of writing and measurement, aims to transform healthcare from a reactive to a predictive model thanks to continuous and real-time reliable patient data measured through wearable technology, both in hospital and at home.”
The SESHAT project arose from needs identified during the COVID pandemic and centers on the remote monitoring of patients with complex care requirements. The project’s goal is to obtain real-time physiological data from patients and have that data help clinicians improve health outcomes. By facilitating better tracking of each patient’s physiological changes, and combining such changes with other available health data, SESHAT project clinicians hope to design and validate better prospective indicators of clinical progress and therapeutic adherence. Their goal is ultimately to help institutions transition from a reactive care model, in which healthcare teams treat symptomatic diseases, to a more proactive model in which teams receive information that enables them to anticipate complications or select more suitable medications to ensure a better therapeutic response, based on each patient’s profile.
“To achieve these objectives,” continued Dr. Estrada, “wearable technology that is robust, reliable, and does not hinder the patient’s normal life had to be selected. After analyzing various options, Masimo technology embedded in medical-grade wearable devices such as Masimo W1 and Radius VSM was chosen. A decisive factor in this choice was Masimo’s willingness to become a technological partner beyond just being a technology provider. We have established a strategic collaboration whereby the SESHAT project will serve as a validation field for technological improvements and identification of new needs in which both institutions will cooperate. When deciding, it was important for the project leaders to work with a leading company in the monitoring technology sector that offers a portfolio with a global solution for all phases of patient care: from the hospital to the home, while also providing integration with Hospital Information Systems.”
The Germans Trias i Pujol University Hospital of Badalona belongs to the Catalan Health Institute. It is a high-tech center that provides health services to a population of 800,000 inhabitants in the North Metropolitan area of Barcelona. Since its foundation, the hospital has been a pioneer in the development and care in various areas of excellence, such as infectious, cardiovascular, inflammatory, oncological, and respiratory diseases, among others. It encompasses a powerful biomedical research campus with 12 internationally renowned institutions, employing over 6,000 professionals and researchers in the field of health. Clinical innovation—transforming clinical problems into opportunities for improving outcomes—is one of the institution’s strategic pillars.
Halo is not FDA cleared and is not available in the U.S.
@Masimo | #Masimo
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2022-23 U.S. News and World Report Best Hospitals Honor Roll.9 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97® and the Masimo W1® medical watch. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius Tº®, Masimo W1 Sport, and Masimo Stork™. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature/.
RPVi has not received FDA 510(k) clearance and is not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo W1™, Radius VSM™, Hospital Automation™, SET®, the partnership of Masimo and Germans Trias i Pujol Hospital (the “Partnership”), and the prospect of SESHAT project. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo’s unique noninvasive measurement technologies, including Masimo W1, Radius VSM, Hospital Automation, and SET®, as well as the Partnership and the SESHAT project ,contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to the assumption that the SESHAT project will last at least three years; risks related to COVID-19; as well as other factors discussed in the “Risk Factors” section of our most recent reports filed with the Securities and Exchange Commission (“SEC”), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contacts
Masimo
Evan Lamb
949-396-3376
elamb@masimo.com